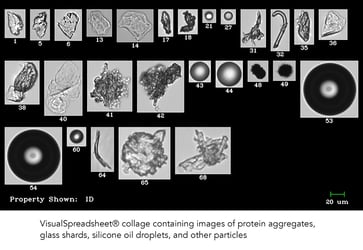
The problem of protein aggregation and presence of extrinsic particles in biopharmaceutical formulations is not a small one. With the improvement of …
Read Post
University of Colorado PhD student, Vaida Linkuviene, along with Fluid Imaging Applications Scientist, Heather Anne Wright, and Co-Director of the …
Read Post

Currently the compendial method for quantifying subvisible particles equal to or greater than 10 µm and 25 µm uses light obscuration (LO), which is …
Read Post

A class of engineered proteins called elastin-like polymers (ELP) have shown promise for advanced drug delivery applications. In order to fully …
Read Post
Proteinaceous particles in parenteral drugs pose an immunogenic risk. These formulations are therefore rigorously characterized for optimal …
Read Post
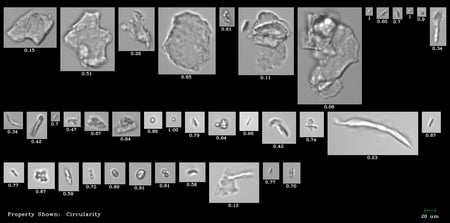
Today’s drug manufacturers are under increasing pressure to understand the particulate composition of their drugs, and to reduce and control …
Read Post
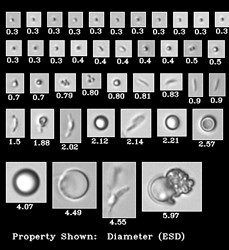
Biopharmaceutical manufacturers strive to ensure patient safety, avoid recalls and protect company reputations. Identifying subvisible particles is …
Read Post
In a recent study by Kiyoshi et al., a Japanese consortium conducted a collaborative study to assess the standardization of flow imaging microscopy …
Read Post
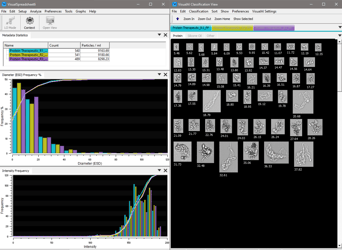
Your current particle analyzer counts the subvisible particles in your protein therapeutic. A particle size distribution curve is generated and …
Read Post

The most common method for characterizing subvisible particles (SVP) ranging in size from 10µm to 100µm is light obscuration (LO). "LO, however, has …
Read Post
Omontys® – a brand name peginesatide injectable – was voluntarily withdrawn from the market less than a year after the product launch. Clinical …
Read Post

AB BioTechnologies, Inc. located in Bloomington, Indiana is a privately held laboratory offering pharmaceutical contract services. Founder and CEO …
Read Post






