
All types of biotherapeutics ranging from protein therapies to cell and gene therapies contain particles. While the types of particles can vary …
Read Post
One of the main values of using flow imaging microscopy (FIM) when analyzing pharmaceuticals is the method's sensitivity to transparent particles. …
Read Post

Assessing subvisible particle content is a required quality control step for biotherapeutics and other parenteral drug products. The United States …
Read Post

Optimizing FlowCam context settings and instrument parameters used during particle analysis is an important step in developing a protocol for …
Read Post

Effective strategies for monitoring particles in biotherapeutics are critical to meet regulatory requirements like USP <788> and mitigate the …
Read Post

Agitation during transportation is one of the most ubiquitous stress conditions to which therapeutic protein formulations and other biotherapeutic …
Read Post

A central challenge with particle analysis in biotherapeutics is the wide size range of particles that may be present. Particles in these samples can …
Read Post

Monitoring particles in biotherapeutics during manufacturing is critical to ensure product quality and to meet regulatory requirements on particle …
Read Post

Comprehensive characterization of biotherapeutics is often challenging given their complexity. In many cases, singular analytical methods cannot …
Read Post
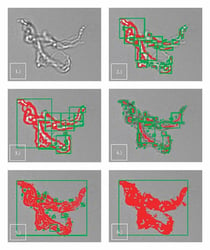
Thresholding is a method of image segmentation used in image processing to discern the boundaries of an object from its background. Semi-transparent …
Read Post
Silicone oil droplets represent a particle type commonly present in protein-based biotherapeutics that are especially prevalent in products packaged …
Read Post
In order to gain the most comprehensive understanding of particles in their samples, researchers often employ several instruments as part of their …
Read Post







